The Link Between Gut Health and Immunity
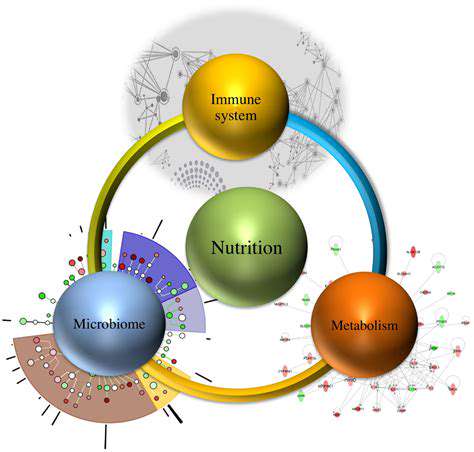
The Interplay of Gut Microbiome and Immunity
Within our digestive tract thrives a bustling metropolis of microorganisms - the gut microbiome. This microscopic community doesn't just aid digestion; it actively shapes our body's defense mechanisms. Scientists now recognize this two-way dialogue between gut bacteria and immune cells as one of the most fascinating biological partnerships in human health. The microbial residents in our intestines train immune cells, helping them distinguish friend from foe while maintaining careful balance.
This symbiotic relationship works both ways. Our immune system constantly monitors and adjusts the microbial population, creating a dynamic equilibrium that's essential for preventing both infections and autoimmune reactions. When this delicate balance falters, the consequences can range from mild digestive discomfort to serious chronic conditions.
How Gut Bacteria Shape Immune Development
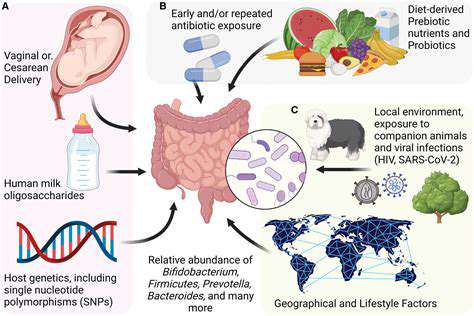
The foundation of our immune system is laid during early childhood, with gut microbes serving as primary instructors. Research shows that babies exposed to diverse microbial communities develop more robust and balanced immune responses. This microbial education helps prevent the immune system from overreacting to harmless substances while maintaining vigilance against genuine threats.
Vaginal birth, breastfeeding, and early environmental exposures all contribute to this microbial education program. These initial exposures create immunological memories that influence health outcomes decades later, highlighting why protecting infant gut health is crucial for lifelong wellness.
Molecular Conversations in the Gut
The gut and immune system communicate through an intricate language of biochemical signals. Microbes produce metabolites that influence immune cell behavior, while immune cells release compounds that shape the microbial landscape. This constant molecular dialogue ensures appropriate responses to dietary components, pathogens, and even our own cells.
Specialized immune cells called dendritic cells act as interpreters in this conversation, sampling microbial products and presenting information to other immune cells. This complex signaling network demonstrates how deeply interconnected our microbial and immune systems truly are.
Nutrition's Role in Gut-Immune Health
Every meal influences the gut-immune axis. Plant fibers serve as fuel for beneficial bacteria that produce anti-inflammatory compounds, while processed foods often promote microbes linked to inflammation. The Mediterranean diet, rich in diverse plants, olive oil, and fermented foods, provides an excellent template for supporting this system.
Emerging research suggests that certain foods may act as immunonutrients, directly influencing immune cell function through their effects on gut microbes. This exciting frontier in nutritional science could lead to targeted dietary strategies for immune support.
When the Gut-Immune Balance Falters
Modern lifestyles often disrupt the ancient partnership between gut microbes and immunity. Antibiotic overuse, ultra-processed diets, and chronic stress can all contribute to dysbiosis - microbial imbalance with far-reaching consequences. This breakdown in communication has been implicated in everything from irritable bowel syndrome to rheumatoid arthritis and even depression.
Scientists are now exploring microbial therapies like fecal transplants and targeted probiotics to restore healthy gut-immune interactions. These approaches aim to reboot the system rather than just suppress symptoms, representing a paradigm shift in treating immune-related disorders.
Gut Microbes: Architects of Immune Function
The Microbial-Immune Tango
Our intestinal tract hosts a microbial ecosystem so complex that scientists often compare it to a vital organ. These trillions of microorganisms don't just coexist with us - they actively participate in training and regulating our immune defenses. The relationship is so intimate that immune cells develop specialized structures just to interact with gut microbes.
This co-evolution has created a system where neither partner can function optimally without the other. Immune cells learn tolerance to food antigens and commensal bacteria while maintaining readiness to attack pathogens. Meanwhile, microbes influence which immune genes get expressed, essentially helping to program our defensive systems.
Critical Windows of Immune Development
The first three years of life represent a crucial period for immune programming. During this time, microbial exposures help establish the immune system's basic operating parameters. Children raised in overly sterile environments or delivered by C-section often show differences in immune development compared to those with more microbial exposure.
This doesn't mean we should avoid cleanliness, but rather highlights the importance of thoughtful microbial exposure. Activities like playing outdoors, interacting with pets, and consuming fermented foods can all contribute to healthy immune education during these formative years.
Diet as a Microbial Modulator
Every bite we take sends ripples through our gut ecosystem. Fiber-rich foods promote bacteria that produce short-chain fatty acids - powerful anti-inflammatory compounds. Polyphenols from colorful plants feed beneficial microbes while acting as antioxidants. Meanwhile, artificial emulsifiers common in processed foods can damage the gut lining, potentially triggering immune reactions.
The timing of food intake also matters. Intermittent fasting appears to benefit gut-immune communication by giving the gut time to repair and reset microbial populations. These findings suggest that both what and when we eat influence immune function through gut microbes.
Microbial Peacekeepers in Immunity
Certain gut bacteria specialize in maintaining immune balance. These peacekeeper microbes produce molecules that calm overactive immune responses while maintaining defense against pathogens. They're particularly important for preventing inappropriate reactions to food proteins and harmless environmental antigens.
When these regulatory microbes decline - due to antibiotics, poor diet, or other factors - the immune system may become more prone to overreacting. This helps explain the dramatic rise in allergies and autoimmune conditions in industrialized societies with altered microbiomes.
Gut-Derived Immune Signals
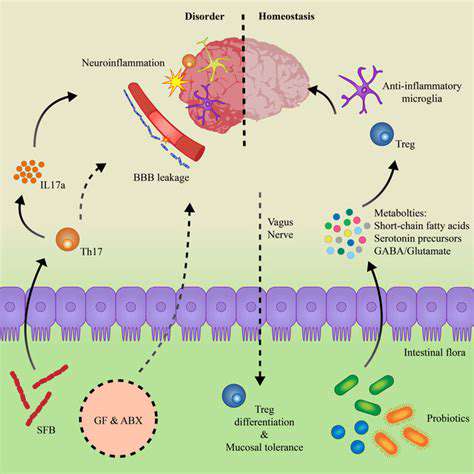
Microbial metabolites travel far beyond the gut, influencing immune cells throughout the body. Some compounds enter the bloodstream directly, while others stimulate nerve signals that communicate with distant tissues. The vagus nerve, often called the gut-brain axis superhighway, also carries immune-modulating messages.
This explains how gut microbes can influence conditions as diverse as skin health, joint inflammation, and even neurological function. The gut truly serves as an immune signaling hub with body-wide influence.
Gut Health as Infection Defense
The Microbial Shield Against Pathogens
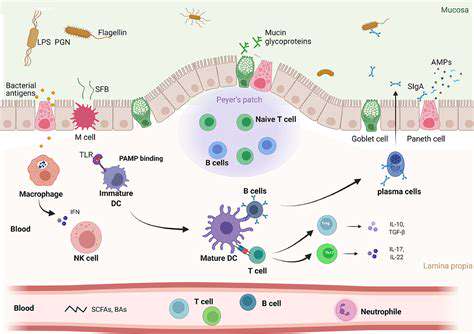
A healthy gut microbiome provides frontline defense against infections through multiple mechanisms. Beneficial microbes physically occupy space that pathogens might colonize, while producing antimicrobial compounds against invaders. Perhaps most importantly, they keep the immune system in a state of educated readiness - prepared to respond quickly to real threats while ignoring false alarms.
This microbial training becomes particularly evident when comparing immune responses in germ-free animals to those with normal microbiomes. Animals raised without microbes show dramatically impaired responses to infections, demonstrating how essential gut bacteria are for proper immune function.
The Inflammation Balancing Act
While acute inflammation helps fight infections, chronic inflammation damages tissues and impairs immunity. The gut microbiome plays a central role in regulating this balance. Certain bacterial strains promote anti-inflammatory responses, while others may trigger more aggressive reactions when needed.
This dynamic regulation explains why gut health influences susceptibility to everything from common colds to serious viral infections. A balanced microbiome helps mount effective responses without excessive collateral damage, while dysbiosis may lead to either overreaction or inadequate defense.
Dietary Strategies for Immune Resilience
Nutritional approaches to infection resistance extend beyond just vitamin C. Diverse plant fibers feed microbial producers of immune-modulating compounds. Fermented foods introduce beneficial strains that may enhance immune surveillance. Even adequate protein intake matters, providing building blocks for immune cells and antibodies.
Emerging research suggests that certain food compounds may specifically enhance antiviral defenses by influencing gut-immune communication. While no single food guarantees immunity, dietary patterns that support microbial diversity appear to offer broad-spectrum benefits.
The Stress-Gut-Immunity Triangle
Chronic stress disrupts gut microbial balance while simultaneously taxing immune resources. The resulting double hit leaves the body more vulnerable to infections. Stress hormones can alter gut permeability, trigger inflammation, and reduce microbial diversity - all factors that compromise immune readiness.
This interconnectedness explains why stress management techniques often correlate with better infection outcomes. Practices like meditation, regular exercise, and adequate sleep all support the gut-immune axis through multiple pathways, highlighting the importance of holistic approaches to immunity.